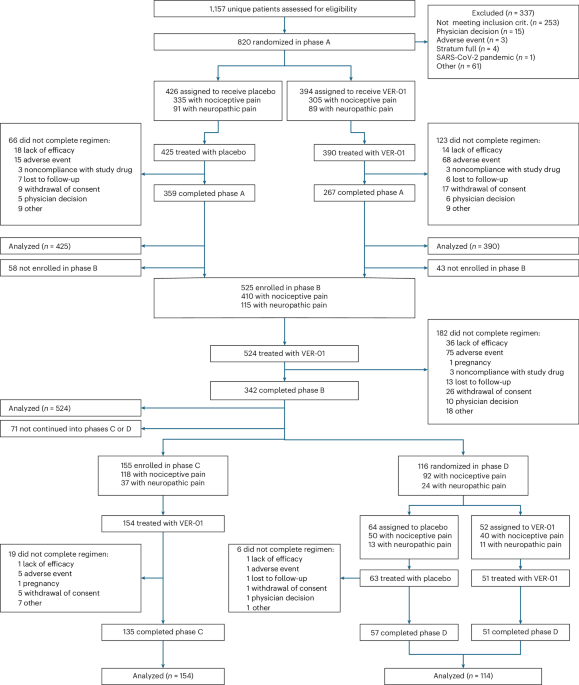
This research investigated the short- and long-term efficacy and security of VER-01 in accordance with suggestions from the European Medicines Company and the US Meals and Drug Administration’s ache pointers 27,38. The check was divided into 4 phases (Determine 2). Part A had a randomized, double-blind, placebo-controlled design of 1 week of break-in to evaluate baseline ache depth, adopted by 3 weeks of titration and 12 weeks of therapy. Part B adopted Part A, affected by a single armored, open label design with a titration interval of three weeks and a therapy interval of 6 months. All contributors coming into Part B had been eliminated as blinding was maintained for Part A all through the research. Members finishing Part B entered both part C or D. Part C confirmed a single arm open label design with a therapy length of 6 months. Part D had a double-blind, randomized withdrawal design with 4 weeks of therapy adopted by 2 weeks of washout. The trial was performed in 66 outpatient websites and university-based hospitals in Germany and Austria, in accordance with the ideas of the Declaration of Helsinki. This trial was authorized by nationwide ethics committees (Supplementary Desk 15) and written knowledgeable consent was obtained from all contributors. No compensation was supplied for participation within the research, aside from refunds for meals and journey bills. The trial was proactively enrolled at ClinicalTrials.gov (NCT04940741) and Eudract (2020-000107-36).
Sufferers had been recruited primarily via doctor workplace notifications, journal advertisements and on-line advertisements. Moreover, researchers collaborating within the research recruited sufferers from present databases. This research enrolled people a minimum of 18 years of age who had been identified with CLBP (decrease again ache for a minimum of 3 months) with or with out neuropathic ache parts. People had been solely included if no particular treatment-able somatic causes of CLBP had been recognized primarily based on an in depth medical historical past evaluation and scientific examination (e.g., vertebral discs). The presence of neuropathic ache parts at baseline was confirmed utilizing a PainDeTect questionnaire with a cut-off rating >18 (Ref. 39). Eligible people will need to have had a ache rating of a minimum of 4 factors on common with an 11-point NRS one month earlier than enrollment and in the course of the apply interval. When treatment was indicated, people had been eligible, and optimized therapy with non-opioid analgesics (e.g., ibuprofen, diclofenac, metamizole, and so on.) was not sufficient to result in ample ache reduction or was inappropriate attributable to contraindications or intolerance. Remedy was thought-about optimized if further dose escalation was medically not possible attributable to potential uncomfortable side effects, or if larger doses had been unlikely to supply further therapeutic advantages.
Members had been allowed to proceed non-pharmacotherapy for CLBP if the affected person had not modified for a minimum of 8 weeks previous to enrollment and if the affected person was prepared to proceed therapy throughout Part A.
If part A was accomplished, contributors had been eligible to be included in part B. Members finishing Part B had been eligible for inclusion in Part D if there was a clinically significant discount in NRS ache rating (≥30%) at baseline at baseline A in Part B.
Members had been excluded if they’d low again ache assessments, extreme psychological sickness (similar to psychosis, schizophrenia, bipolar dysfunction), or different painful comorbidities that doubtlessly interfered with a historical past of alcohol, medication, or treatment abuse. Moreover, contributors who used cannabis-based drug merchandise inside 30 days of screening had been excluded. An entire listing of inclusion and exclusion standards might be discovered within the Supplementary Notes (Qualification Standards).
The analysis product VER-01 is a standardized full-spectrum extract derived from the patented hashish Sativa L. pressure DKJ127. The plant substances of ver-01 are made up of dried C. sativa dkj127 L. flowers that haven’t been frag-folded. Full-spectrum extracts of plant substances are manufactured in a superior manufacturing (GMP) licensed facility and standardized to five% tetrahydrocannabinol (THC).
Every dose unit (119 µL) of the completed product VER-01 accommodates 50 µL of full-spectrum extract, supplying 2.5 mg THC, 0.1 mg cannabigerol, and 0.02 mg cannabidiol as excitation materials.
Moreover, VER-01 accommodates a posh and well-characterized combination of terpenes, flavonoids, carotenes, phytosterols, and different bioactive compounds.
Placebo contained sesame oil, hashish scent and colorants to imitate the looks and sensory properties of VER-01.
Analysis merchandise had been supplied in 30 ml amber glass bottles and had been taken verbally with a dosage syringe, following the suggestions of the European Ache Federation, advocating oral preparation within the administration of continual ache.
This research included two randomizations carried out firstly of phases A and D. Eligible contributors had been randomly assigned at a 1:1 ratio for VER-01 or placebo. Randomization was stratified based on the presence of neuropathic ache parts. Members, investigators, and analysis web site employees had been hidden in therapy assignments. VER-01 and matching placebo had been distributed in the identical amber glass bottle.
Randomization was carried out in 4 blocks utilizing a computer-generated randomization listing. The dimensions of the randomized block has not been disclosed to the investigator.
Throughout the screening go to, contributors had been supplied with editors and instructed about their use. Most rankings had been recorded each day by the editor, whereas others had been recorded on paper on the examination web site after which transferred to an digital knowledge seize system by the positioning consultant. At go to A2 go to, eligible contributors had been randomized. Members had been suggested to take analysis treatment constantly with or with out meals. The person optimum doses of contributors (each VER-01 and placebo) had been decided in the course of the 3-week titration interval, following the written titration scheme. If the analysis product was effectively tolerated, the dose was elevated each 3 days with one dose unit within the night and one dose unit within the night till contributors skilled ample symptom reduction or reached the utmost dose of 13 doses. Morning and night doses had been adjusted independently.
Taking painkillers apart from rescue medication was banned over the past three weeks of Part B, Part B and Part D. Using rescue drug (ibuprofen) was restricted to a each day dosage of two,400 mg, as much as three days per week. When ibuprofen was contraindicated, paracetamol was supplied at a most each day dose of 4,000 mg. Members weren’t permitted to provoke non-pharmacotherapy throughout part A or the 9 weeks previous to inclusion in part D. If ongoing non-pharmaceutical remedy was already in place, adjustments needed to be continued via participation in Part A and D.
Members used editors to document dose, ache depth (11 factors NRS), sleep high quality (11 factors NRS), and rescue treatment use in any respect research levels. Moreover, contributors used the editor to document potential withdrawal signs throughout part D utilizing CWS.
Part A included 6 visits (Fig. 2) – display (A1), randomization (A2), termination of titration (A3), and three visits at 4-week intervals: therapy stage (A4-A6). Part B consisted of 4 visits. Three visits are made at two months intervals, on the finish of 1 titration (B7) (B7) and the therapy stage (B8-B10). Part C consisted of three visits and follow-up visits (C14) at two-month intervals in the course of the therapy part (C11–C13). Part D included two visits on the finish of therapy (D11) and follow-up go to (D12).
Security assessments embody AES, laboratory parameters, very important indicators, bodily examination, urine drug screening, being pregnant checks, 24-hour ECG evaluation (a subset of 120 contributors), and substance dependence evaluation based on ICD-10 (F12.2) standards. Investigators assessed the severity and causality of AES and documented the initiation, length, severity, and habits and coverings carried out.
The connected protocol offers a whole analysis schedule.
The first endpoint for Part A was fifteenth week from the common weekly ache depth CFB (week-1) within the morning, measured with 11-point NRS (0 = “no ache”, 10 = ‘imagining the worst ache’). The first secondary endpoint for part A was the CFB of the full NPSI rating for part A (entry A6) for contributors with neuropathic ache parts. The NPSI contains 10 gadgets measuring the severity of spontaneous ache (4 gadgets), painful assaults (three gadgets), induced ache (two gadgets), and irregular sensations (one merchandise). Every merchandise was scored with an 11-point NRS. Complete scores vary from 0 (no signs) to 100 (worst conceivable signs).
The first endpoint for part D is the time of therapy dysfunction, the first endpoint for part D, outlined as a 7-day imply of morning ache depth within the NRS, a rise of ≥20% and ≥1 level, in comparison with part D baseline week 44.
In any respect research levels, the proportion of contributors who achieved ache reduction of ≥30% and ≥50% had been assessed, reflecting outcomes thought-about clinically significant 22,23,42.
Extra diary-based secondary efficacy endpoints assessed in any respect research levels included adjustments in imply ache depth (11-point NRS), rescue treatment consumption, sleep high quality of 11-point NR (0 = “unaffected”, 10 = “absolutely affected”), and the proportion of contributors with ≥30% and 50% enchancment in sleep high quality.
Go to-based secondary efficacy endpoints assessed in any respect research levels included CFB for NPSI whole scores and PGIC on a 7-point Likert scale. “What about decrease again ache in comparison with earlier than collaborating within the research?” (0 = 6 = ‘very dangerous’ than ‘superb’) and high quality of life assessed within the Quick-Time period Well being Survey 36v2 (SF-36) 43,44. SF-36 included 36 questions on bodily and psychological well-being and had been rated primarily based on eight area scores (bodily operate, function system, bodily ache, normal well being, vitality, social operate, function feelings, psychological well being). Scores ranged from 0 (most fault) to 100 (no fault).
Secondary effectiveness endpoints primarily based on the next go to had been additional evaluated in Research Part A. The severity of incapacity was assessed at baseline and at end-of-treatment RMDQ 45. The RMDQ is a 24-item questionnaire assessing the impact of decrease again ache on purposeful exercise, with scores starting from 0 to 24, with larger scores indicating larger incapacity. Moreover, sleep high quality was measured utilizing the Medical Outcomes Analysis Sleep Scale (MOS-SS), consisting of 12 gadgets that assess sleep notion and upkeep, respiratory issues throughout sleep, length of sleep, sleep validity, and daytime somatic validity. The MOS-SS score was primarily based on two sleep drawback indicators. The upper the sleep rating, the higher the scientific outcomes.
Security was assessed primarily based on the incidence of AEs, together with severity, severity and relationship with the analysis drug. Moreover, affected person satisfaction with tolerability was assessed per go to utilizing a 5-point Likert scale. Drug dependancy and potential for abuse had been measured on Go to base utilizing ABC, a 20-item guidelines used to observe indicators of ADICTION 47. ABC whole scores of three or larger point out inappropriate drug use. Withdrawal signs had been recorded each day by the editor utilizing CWS throughout therapy in Part D and through washout in Part C and D. The CWS consists of 19 gadgets representing potential withdrawal signs related to hashish withdrawal. Every merchandise is rated on a scale of 0-10, with 0 indicating “nothing in any respect” and 10 indicating “very” 48.
To reveal the benefit of VER-01 over placebo on the major endpoint of Part A, a complete of 732 contributors needed to be randomized at a 1:1 ratio. Pattern dimension calculations had been primarily based on the anticipated therapy distinction of 0.6 nRS factors, SD of two.5 factors, and two-tailed significance ranges of statistical energy of 5% and 90%. For the first secondary endpoints in Part A, 180 contributors with neuropathic ache parts needed to be randomized at a 1:1 ratio. This calculation was primarily based on the assumed therapy distinction of 10.5 NPSI factors, SD of 25.0, two-sided significance stage of 5% statistical output and 80% statistical output. Primarily based on the 22% share of contributors with neuropathic ache parts, these assumptions resulted within the whole pattern dimension of Part A to 808 contributors. Eligible contributors who had been already within the break-in stage had been nonetheless allowed to be randomized.
Demographic knowledge and different Part A baseline traits had been summarized individually throughout therapy teams for every research part primarily based on the outlined set of analyses.
The first endpoints for part A had been examined by evaluation of covariance fashions, with therapy used as important results and baseline traits (presence of neuropathic ache parts, NRS morning ache depth, age, gender, nation) as covariates. The evaluation was primarily based on attribution knowledge when inter-occurrence occasions prevented knowledge assortment, or when knowledge collected after the incidence of the occasion that occurred skewed the interpretation of the outcomes. Relying on the kind of inter-occurrence occasion, knowledge had been attributed assuming they lacked randomly lacking (MAR) or non-random (MNAR). When the information was MAR, these had been attributed utilizing a number of project (MI) fashions primarily based on accessible knowledge from comparable contributors in the identical therapy group. If the information is MNAR (e.g., early abortion attributable to AES or lack of effectiveness), the information had been attributed beneath a conservative method of “leaping to reference.”
This method was thought-about conservative because it assumes that contributors discontinuing VER-01 would expertise comparable outcomes to placebo arm outcomes, no matter the advantages of prior therapy. Proof from focus machine trials 49,50,51 means that the analgesic impact achieved previous to therapy discontinuation often lasts for a number of weeks and doesn’t trigger ache greater than placebo. Moreover, contributors who discontinue aggressive therapy might proceed to learn from non-specific trial results, together with constructive expectations, scientific monitoring, and enhanced behavioral adjustment, that are equally current within the management group. Lastly, the character of AES that encourages discontinuation is estimated to be delicate to reasonable and momentary, worsening the result of everlasting ache than placebo.
MI primarily based on the MNAR assumption was carried out utilizing the proposed placebo MI algorithm within the references. 52. Basically, a two-stage MI method was adopted. In step one, we calculated an MCMC project mannequin with 100 attributions to transform any lacking knowledge patterns into monotonous lacking patterns. Within the second MI step, a monotonous regression mannequin was calculated with these 100 attributions. The estimated parameters had been mixed based on Rubin guidelines. BOCF and Locf Inputs had been carried out as supportive analyses.
The first secondary endpoints had been evaluated equally to the first endpoints in Part A utilizing evaluation of the covariance mannequin. The identical project technique was utilized as the first endpoint. Security was assessed primarily based on the incidence of AEs in submit hoc comparisons carried out utilizing the double-sided chi-square check.
It was deliberate to incorporate 500 contributors in Part B to gather efficacy and security knowledge from a minimum of 300 contributors over six months. Moreover, 150 contributors had been included in Part C to gather long-term efficacy and security knowledge for a minimum of 100 contributors over 12 months.
The calculation of the pattern dimension for part D is assumed to be a 25% therapy failure price for VER-01 and a 55% for placebo, and is predicated on the bibliography. 53. A log-rank check evaluating two survival curves for time to therapy dysfunction required a pattern dimension of 78 (39 per 39) to attain 80% statistical energy beneath a two-sided significance stage of 5%.
The first endpoints for part D had been analyzed as covariates as therapy and baseline traits as important results (presence of neuropathic ache parts, NRS ache depth at 43 weeks, presence of age and gender). Occasions indicating therapy failure (similar to overdose of rescue treatment, potential to face up to, lack of efficacy, or discontinuation of the research attributable to violation) had been categorized as occasions of therapy failure. Knowledge from contributors with present occasions that didn’t point out therapy failure had been thought-about lacking and attributed primarily based on MAR assumptions. Censorship was carried out in time-to-event evaluation because of the cancellation of the research for causes not indicating therapy failure.
The evaluation features a part A and D evaluation set. All randomized contributors who acquired a minimum of one research drug at every stage had been included, and contributors had been assigned to therapy teams as randomised (full evaluation set) or therapy (security evaluation set). Security evaluation features a set of phases B and C. All contributors who acquired a minimum of one research drug at every stage had been included. No impartial knowledge monitoring committees had been used. All statistical analyses had been performed by an impartial scientific analysis institute utilizing SAS software program model 9.4 (SAS Institute).
For extra details about analysis design, see the overview of the Nature Portfolio report linked to this text.