That appeared like excellent news. Years after it turned obtainable in different international locations, the highly effective Cell remedy for kind 1 diabetes Authorized within the US in June. it supplies That is the primary time that sufferers in america will obtain transplants of insulin-producing islet cells exterior of a medical trial, a remedy that might result in insulin independence in some circumstances.
However the specialists who helped invent and develop islet transplant surgical procedure aren’t celebrating. Moderately, it criticizes the U.S. Meals and Drug Administration (FDA) for selecting to control transplanted islet cells as medicines slightly than organs. In consequence, a single non-public firm would be the solely accredited U.S. provider of islets for transplantation.
Say “I do not perceive the logic” Dr. Camilo Ricordidirector emeritus of the Diabetes Institute, on the choice to control pancreatic islets as medicines slightly than organs. “There isn’t any scientific foundation for that.”
Piotr Witkowski, MDstated the director of the pancreas and islet transplant program on the College of Chicago. Nobody who understands transplant rules helps this (regulation) in America or anyplace else on the planet. ”
In feedback to Diabetes Every day, the FDA supported its determination to approve the remedy, however didn’t tackle the bigger query of whether or not pancreatic islets must be regulated as medicines. CellTrans, the corporate accredited by the FDA to distribute islets for transplantation, didn’t reply to a number of requests for remark.
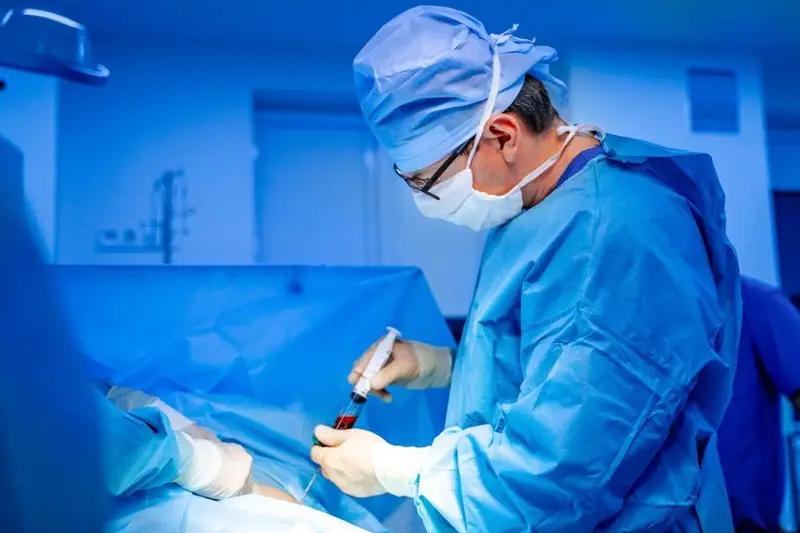
Pancreatic islet cell transplantation
Islet cell transplantation is a sophisticated remedy for kind 1 diabetes. Briefly, a health care provider takes a donor’s islets of Langerhans (a inhabitants of cells within the pancreas that features insulin-producing beta cells) and injects them right into a diabetic affected person, normally the liver. If profitable, sufferers can both cease utilizing insulin utterly or considerably cut back their dependence on insulin.
Some transplant recipients Haven’t obtained insulin remedy for a few years Nevertheless, defending the brand new cells from the physique’s immune system requires anti-rejection medication, which include their very own important dangers.
Experiments are underway to Evaluating the usage of laboratory-grown islet cellsNevertheless, at present the islet cells utilized in these transplants have to be harvested from the pancreas of a deceased organ donor. As a result of these donor cells are uncommon, the variety of surgical procedures that may be carried out is proscribed. However for sufferers with pressing wants, similar to those that have excessive blood sugar management difficulties or are unaware of their hypoglycemia, islet transplantation could be a lifesaver.
regulatory confusion
Shortly after the primary profitable islet transplant in 1993, the FDA introduced that the transplanted islets could be handled like islet transplants. drugs Moderately than organs or subparts of organs. This determination puzzled specialists. “We’re the one nation with such a regulation,” says Dr. Ricordi, who developed it in 1988. Islet cell isolation expertise That is what made the transplant potential.
The issue, Ricordi explains, is that pancreatic islets taken from donor our bodies do not meet the requirements of precision and consistency anticipated of pharmaceutical components. Much like different organs, islet cells can’t be precisely assessed for sterility, purity, or efficiency previous to transplantation. Even when it have been potential, the nonprofit analysis hospital that developed the remedy merely would not have the assets to fulfill the FDA’s expectations.
“No educational establishment can spend tens of millions of {dollars} and years making an attempt to beat this drawback. B.L.A. (biologics license utility),” Ricordi stated.
Through the years, Dr. Witkowski, Ricordi, and plenty of of their colleagues marketing campaign It was named the “Island for U.S. Cooperation” to alter the FDA’s considering. “We’ve instructed the FDA that affected person security is in danger if islets are accredited as medication and distributed completely by business firms. It must be regulated for that cause,” Witkowski stated.
College and non-profit hospitals couldn’t justify the associated fee; Celltrans They raised sufficient cash to get via the FDA’s web. However initially, it was unclear whether or not CellTrans may overcome the inevitable consistency issues inherent in human organs.
Within the center April 2021 Public Listening toan FDA advisory committee evaluated CellTrans’ medical trial outcomes. Professor Witkowski stated the presentation confirmed what transplant specialists already knew: islet cells are merely harvested from a beating corpse. cannot do it Meets FDA pharmaceutical manufacturing requirements. Not surprisingly, the FDA discovered no correlation between islet high quality measurements and medical efficacy, one thing unpredictable that may be thought-about unacceptable for many different medicines. .
“The FDA clearly defined why CellTrans failed,” Riccordi asserts. Nonetheless, the vast majority of the panel’s impartial specialists agreed that the remedy had an “total favorable benefit-risk profile for some sufferers with kind 1 diabetes.”
The committee’s help didn’t speed up CellTrans’ remedy towards approval. The method had been in regulatory limbo for about two years.
stunning approval
Witkowski stated in June that U.S. Sen. Mike Lee (R-Utah) drafted a legislative effort to amend rules and pave the way in which for authorized islet cell transplants in america. I felt that the pancreatic islet cooperation was lastly on monitor. of island legislation It promised to “transfer the island to a extra applicable regulatory framework.” Nevertheless, Witkowski’s optimism didn’t final lengthy.
Every week after Sen. Lee introduced his invoice, the FDA introduced it had accredited Celltrans’ cadaveric islets. The brand new island supply is called Donislesel (Rantidra). The Isles for US Collaborative responded with suspicion: states The transfer “considerably complicates the trail to passing the ISLET Act and implementing necessary regulatory updates.”
CellTrans needed to spend tens of millions of {dollars} to efficiently apply for a BLA, however critics argue that the enterprise is not really growing something new. a 2021 letter in transplant worldwide “It is only a new identify for islet allotransplantation,” Lantidora stated.
Witkowski agrees: they did not change something. This isn’t a modified human organ, however they name it a drug and promote it as a drug. ”
Lantidra is at present the one FDA-approved supply of islets for transplantation to deal with kind 1 diabetes.
Islet transplantation and dangers
Islet cell transplantation has nice potential to deal with kind 1 diabetes. Lantidra’s most necessary trial was performed at a tutorial transplant heart following all recognized protocols, with 30 % of contributors reaching at the least 5 years of insulin independence.
Nevertheless, islet transplantation is related to regarding unwanted side effects, primarily because of the highly effective immunosuppressive medication required to guard the brand new cells. A whopping 87 % of Lantidra trial contributors skilled at the least one “extreme” response, and 27 % skilled at the least one “extreme” response. threaten life Facet reactions. There have been 211 separate infections in 30 sufferers. One topic died from an an infection that led to sepsis and a number of organ failure, and the opposite suffered life-threatening liver lacerations.
“This wasn’t a shock to us,” Witkowski stated. There are important dangers inherent in islet transplantation, which is one cause why this process is reserved for sufferers with probably the most extreme glycemic management issues.
Nevertheless, Witkowski is worried that distributing Lantidra as a drug would disrupt the chain of duty for maintaining organ transplants protected, and that Lantidra’s danger profile may worsen in the true world. There’s.
Historically, islet transplant surgeons are accountable for all elements of the surgical procedure, together with organ choice and analysis, efficiency of the surgical procedure, and ongoing monitoring of the affected person.
“I take duty for all the things,” Witkowski says. “Donor choice and all the things. If something goes improper, it is as much as me and my transplant heart to reveal the outcomes.”
However by distributing Lantidora as a drug, “surgeons lose management of the product. They haven’t any selection. They must take what they’re given.”
CellTrans might also select to promote Lantidra solely to nonprofit transplant facilities and place it within the arms of probably the most certified surgeons. Nevertheless, there may be additionally the likelihood that the island may very well be bought to a personal facility, ignoring the standard community of transplant facilities. Witkowski is especially involved about this latter risk. Non-public clinics, which aren’t required to reveal outcomes, might have “no accountability and no subsequent oversight.”
“They will select to do it the proper manner, however they don’t seem to be obligated to do it the proper manner.”
moral issues
Writer of 2021 transplant worldwide A letter composed of European endocrinologists, immunologists, and transplant surgeons decried the moral implications of approving Lantidra as a drug.
The switch of selling rights for the isolation of islets to personal business firms may foresee the commercialization of human organs and their subparts… This raises severe authorized and moral points as a result of it’s obtained primarily based on “unremunerated donations, donor altruism, and solidarity between donor and recipient.”
Specialists additionally outlined different points. They argued that Lantidra’s approval would doubtless stifle competitors and restrict the remedy to the wealthiest sufferers. “Extreme regulatory burdens which can be unwarranted by scientific proof may irreversibly impede their utility and additional growth by growing prices and limiting entry.”
Witkowski has related issues. He defined that organ transplant ready lists are managed dynamically by the group. Unified community for organ sharing (UNOS) is a nonprofit group devoted to the equitable distribution of organs primarily based on affected person want. However as a personal firm, CellTrans “can select who it needs to serve.” Though these islands are eligible for insurance coverage reimbursement, firms have sturdy incentives to cost a excessive worth for Lantidra, doubtless placing it out of attain for some sufferers who want it.
CellTrans was granted seven years of exclusivity underneath the Orphan Medicine Act, however the enterprise formally We’ve dedicated to waiving our exclusivity rights and permitting different firms to submit BLAs and take part as accredited island suppliers. However Witkowski identified that CellTrans may simply revoke exclusivity.
“I am very disenchanted.”
Lantidora’s drug approval seems to run counter to the custom of nonprofit collaboration that helped develop islet transplantation within the first place.
When Ricordi invented a way to isolate islet cells (the approach CellTrans wanted for Lantidra), he shared it freely. “I’ve launched all of my mental property to the world. I wish to deal with kind 1 diabetes within the quickest and most effective manner potential, waive royalties, and have entry to gear, drawings, and coaching. We’re proud to have developed it and shared it with the world to make it potential.”
With out coming throughout as disrespectful, Ricordi defined that he was impressed by the scientists who found insulin. Nobel laureate Frederick Banting and his colleagues Offered insulin patents for $1.00 every It was based within the Nineteen Twenties to make sure that life-saving medicines may very well be distributed as shortly and affordably as potential.
In a merciless irony, Ricordi’s improvements are actually used completely in america by business enterprises primarily unrelated to him.
“It’s extremely disappointing to see a lot effort put into staying nonprofit, after which all the things being swept away to the for-profits due to this outdated FDA regulation.” I am not criticizing them. They performed by the principles. …However I want the FDA would give attention to extra severe points.”
Witkowski is not certain what’s going to occur subsequent. He’s hopeful that Sen. Lee and different members of Congress who pledged to help the ISLET Act is not going to again down.
“However we do not know what is going on to occur. I do not assume they anticipated this sort of response from the FDA. We’ll look ahead to them to tell us if they are going to surrender or preserve preventing.” ”
Passing this invoice would require bipartisan cooperation. That’s briefly provide in a divided political atmosphere.
Professor Witkowski stated, “If we go the Islet Act, pancreatic islets will grow to be a nationwide useful resource like every other organ and might be protected by legislation.”
(Translate tag) Beta cells